LOADING
Share

The purpose of this article is to outline the major challenges faced in work with pregnant women who are misusing substances and describe the elements of a successful comprehensive care model that has been implemented in the UNC Horizons program in the Department of Obstetrics and Gynecology at the University of North Carolina at Chapel Hill.
The following case study, although fictional, is illustrative of many substance-using women who become pregnant. Most substance-using women have a strong desire for a healthy child, but many are extremely socially disadvantaged and have no place to turn to help them reach their goals and human potential. They feel, often rightly, that society considers them the most reprehensible of social outcasts. Research has consistently shown that it is possible to make a substantial difference in the lives of many of substance-using women, including allowing the delivery of healthy, full-term infants. However, research indicates that positive outcomes for this population require comprehensive care programs that carefully coordinate care from multiple service providers—including obstetricians, psychiatrists, neonatologists, pediatricians, nurses, psychologists, counselors, social workers, and child care workers—all working towards the common goal of health and wellbeing of the mother and her fetus, and also of the larger family unit, including other children, the partner of the expectant mother, and even her extended family.
Case Study: Angela Smith
Angela Smith presents at the UNC Horizons program on referral from a current UNC Horizons patient. Angela is twenty-four years of age, with a singleton pregnancy of sixteen weeks estimated gestation. She has evidence of venipuncture stigmata on both arms and provided a urine sample positive for both opioids and marijuana. She reports that she is HIV-negative and HCV-positive. She appears underweight, although in otherwise good health, and in need of dental services. Past history indicates two previous live childbirths. She reports five emergency department visits following incidents of either sexual and/or physical abuse in the past twelve months.
Angela has not yet seen an obstetrician. She reports that she saw an obstetrician on several occasions during her first pregnancy, but that the visits made her felt terrible about herself, because he treated her “like a drug addict.”
Family History
Angela reports that both her mother and father have smoked cigarettes her entire life. She indicates that both parents have histories of heavy alcohol use. She reports that they often argue, particularly after heavy bouts of drinking, and her father will often hit her mother during these arguments. She indicates that she was often the object of her father’s physical abuse during these times, so learned at an early age to avoid her parents when they were drinking.
She denies any sexual abuse from either parent; however, she indicates that her uncle episodically sexually abused her beginning around age six until he moved from the area when she was thirteen. She states she did not report the sexual abuse to her parents because she feared she would be beaten for “lying” or “encouraging” such behavior on the part of her uncle.
Psychosocial History
Substance use began with cigarette smoking at age fourteen, followed by alcohol use at age fifteen. Angela reports she began drinking only on weekends with both girls and boys her age or older, and often binge drank. She reports that she now smokes “a pack a day” and drinks episodically with her boyfriend, generally on weekends, during which times she will binge drink.
She was introduced to sex by a boy two years older during one of these weekends, when she was “drunk.” Sexual activity was episodic throughout the remainder of high school, with no use of birth control or disease protections. She became pregnant for the first time at age eighteen, during her senior year in high school. She gave birth shortly after graduation, at which time her parents “threw her out of the house.” She gave this child up for adoption, a cause of both sadness and loss six years later. She gave birth to a daughter, Ellen, when she was twenty. Angela lost custody of Ellen at birth, when she was reported to child protective services by the delivery hospital given her use of heroin during her pregnancy. She was subsequently able to regain custody after showing her protective services worker she was “clean.” However, she again lost custody of Ellen approximately six months ago, following reporting a domestic dispute with her boyfriend, who had hit and threatened her.
In terms of the current pregnancy, Angela reports that she stopped drinking as soon as she found out she was pregnant, but has been unable to stop using either heroin or marijuana. She lives in the apartment of her current boyfriend, who physically abuses her when he experiences withdrawal symptoms from his misuse of prescription opioids. He also threatens to beat her if she attempts to deny him sex. She has been arrested for prostitution on two separate occasions, which she reports occurred before she met and moved in with her current boyfriend. She has no other history of employment. She reports she has no close friends, and her social contacts are with substance-using peers.
She indicates she came to Horizons because she wants to have a healthy child. She believes that a child will strengthen her relationship with her boyfriend, and lead him to be a more responsible provider and parent.
Psychiatric/Psychological History
Angela has had no contact with any form of professional counseling. She reports she was “diagnosed” with depression by her primary care provider at age sixteen when she revealed thoughts of self-harm to him. She was given a prescription for an SSRI, but never filled the prescription because her mother refused to pay for the medication, saying that Angela “just felt sorry for herself.” She attempted suicide twice, trying to overdose on opioid pain medication once at age nineteen and another time at age twenty-one. She reports no thoughts of harm to herself or others.
Substance Use in Pregnant Women
Women who continue to misuse substances after finding out that they are pregnant deserve help and compassion. As Figure 2 illustrates, data from the combined 2012 and 2013 National Survey on Drug Use and Health show that pregnant women use both licit and illicit substances (SAMHSA, 2013, 2014b). With the exception of nonmedical use of psychotherapeutics, the rates of substance use have remained stable among pregnant women for the past decade. The Treatment Episode Data Set (TEDS) also shows that the proportion of pregnant substance abuse treatment admissions remained stable between 2000 and 2010 (4.4 and 4.8 percent, respectively); however, there was a decrease in the proportion of pregnant women reporting alcohol misuse (with or without drug abuse) and an increase in the reporting of drug abuse (US Department of Health and Human Services, 2009).
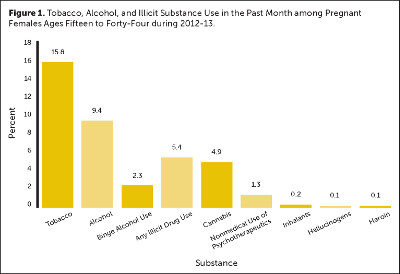
The increased health risks associated with tobacco use are well known. A recent epidemiological study of the issue found that maternal cigarette smoking resulted in an increased risk of intrauterine death, preterm delivery, lower birth weight, respiratory distress, cardiac malformation, and neonatal death relative to nonsmoking control mothers. They conclude that “Any amount of daily smoking” on the part of the mother “appears to harm the fetus and newborn” (Mei-Dan et al., 2014).
Similarly, the negative impact of alcohol on fetal development is now known as fetal alcohol spectrum disorders (FASD) that include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD) (SAMHSA, 2014a). FAS is the most severe form of FASD, and presents lifelong problems to the child, beginning at birth. Infants born with FAS often show a constellation of physical signs marked by abnormal facial features, growth problems, and central nervous system problems. They may have hearing and/or vision problems. As they develop, they may show enduring learning, memory, attention, and/or communication and socialization deficits.
Children with ARND typically have lifelong cognitive and behavioral deficits milder than FAS individuals. Infants with ARBD are often born with cardiac, kidney, bone, and/or hearing defects. Although the general conclusion is that “There is no known amount of alcohol that is safe to drink while pregnant. There is also no safe time to drink during pregnancy and no safe kind of alcohol to drink while pregnant” (SAMHSA, 2014a), there are some studies showing that low to moderate drinking is not associated with adverse child effects up to age five (Falgreen Eriksen et al., 2012; Kesmodel et al., 2012a; Kesmodel et al., 2012b; Skogerbø et al., 2012; Underbjerg et al., 2012). Thus, the World Health Organization has stated that “The level of maternal consumption that produces fetal alcohol spectrum disorders (FASD) has not been established and is influenced by genetic and other maternal and fetal characteristics” (2014). Finally, it should be noted that postpartum use of alcohol on the part of the mother, leading to problems in parenting and caring for the child, can produce its own negative consequences for mother, child, and family.
The short- and long-term effects of in-utero exposure to most illicit substances on both fetal and childhood development require more research. Repeated exposure to certain classes of volatile agents (e.g., inhalants), typically toluene—found in organic compounds as diverse as gasoline, paint thinners, and nail polish—are thought to cause fetal solvent syndrome (FSS), with deficits similar to those seen in FAS (Jones & Balster, 1998). Less frequent use of toluene or use of other volatile agents raise the risk of miscarriage and premature birth. There also appears to be an increased risk for problems in brain development that leads to developmental delays and learning problems in elementary school (Jones & Balster, 1998).
Fetal, infant, and childhood effects of marijuana exposure are poorly understood, and the research on the topic is mixed. Until recently, the general conclusion was that continued in-utero exposure to cannabis leads to an increased risk of a low birth weight infant, the possibility of mild withdrawal symptoms at birth, what appear to be short-term problems with autonomic regulation, and an increased risk of early childhood problems with verbal learning, memory, and attention (Fried, 1995). The impact of marijuana on cognition in infants appears inconsistent, although there does appear to be a possible subtle impact on both cognition and behavior during childhood (Huizink, 2014). Indeed, recent pharmacological research in both mice and human fetuses suggests that prenatal exposure to cannabis may rewire fetal circuitry given the possible identification of a molecular target for cannabis during corticogenesis, the process in which the cerebral cortex is created (Tortoriello et al., 2014).
Maternal use of cocaine has a particularly checkered history in the research literature and mainstream press. Cocaine was an issue of considerable attention in the 1980s and 1990s. Stimulated in part by an article by Chasnoff and colleagues (Chasnoff, Burns, Schnoll, & Burns, 1985), that raised concerns regarding a significant deleterious impact of prenatal exposure to cocaine on the fetus, scholarly and political forces of the time promulgated the idea of a “lost generation” of “crack babies” whose medical care costs would be outsized, and who would overwhelm school systems because of what was widely thought to be their wide-ranging and severe lifelong learning, emotional, and behavioral deficits. Such arguments are widely believed to have led to an increase in funding for the federal war on drugs. Moreover, they led to both the criminalization and prosecution of mothers who used cocaine during their pregnancies, including charges of attempted murder and child endangerment. These laws, colloquially termed “cocaine mom” acts, have subsequently been extended in several states—Wisconsin, Oklahoma, Minnesota, and South Dakota—to cover illicit substances and alcohol, leading to civil confinement or criminal prosecution. Fortunately, recent research has suggested few if any long-term consequences of prenatal exposure to cocaine are not as serious as previously thought (Fallone et al., 2014).
Although use of heroin and other opioids during pregnancy appears to be at such low levels as not to merit attention, the converse is actually true, as the public health implications of maternal opioid use are enormous. Maternal use of heroin or nonmedical use of opioid analgesics led to in utero exposure of approximately 53,400 neonates in 1992 (NIDA, 1996). Viewing the issue from the treatment perspective, it is notable that in 2008, 15 percent of pregnant women admitted to substance use treatment tested heroin-positive, and 14 percent admitted to using prescription analgesics nonmedically (US Department of Health and Human Services, 2009).
Although precise figures are lacking, it is conservatively estimated that more than 50 percent of infants prenatally exposed to opioids will experience neonatal abstinence syndrome (NAS), a constellation of signs and symptoms indicative of central nervous system dysfunction as well as problems with neonatal gastrointestinal, respiratory, and the autonomic nervous systems (Jones et al., 2012). Hospital costs associated with treatment of a neonate for NAS were estimated to be $53,400 in 2009, with 78 percent of these charges paid by Medicaid.
Summary
It seems logical that prenatal exposure to licit and illicit substances raises the risk level for the child. However, research that is able to both isolate and quantify such effects is largely absent. Consequences of prenatal exposure to a particular substance are confounded by multiple other hazards to which the fetus and then the child can be exposed. Few published studies have been able to quantify the amount of a substance to which the fetus has been exposed, or the trimester during which such exposure occurred.
Moreover, there exists the multiplicity of environmental factors that could moderate such relationships, including, during the prenatal period:
- Maternal health
- Exposure to sexually transmitted diseases
- Maternal nutrition
- Amount and quality of prenatal care
- Maternal exposure to abuse and violence
Following birth, the environmental factors could include:
- Emotional, physical, and/or sexual abuse of the infant and child
- Diet and nutrition
- Attentive care and nurturing
- Cognitive stimulation
- Socioeconomic factors
Importantly, the synergistic effects of the use of other licit and illicit substances may serve to exponentiate or dampen the impact of a particular substance. Finally, there is the need to consider the role of protective factors, such as child resilience or secure attachment in mitigating the adverse impact of a particular substance on the fetus and child. As one example, Bada and colleagues found that high levels of prenatal cocaine exposure paired with maternal use of other substances at a high level was a significant risk factor for behavioral problems during adolescence (Bada et al., 2012). However, this relationship was attenuated in the presence of protective factors.
Treatment
Treatment for pregnant women with substance use disorders can broadly fall in two categories: medication and behavioral, recognizing that the combination of both treatment types is often most effective if medication is available and appropriate. A wide range of evidence-based behavioral treatments exist, and are used for treating addiction disorders involving substances as diverse as marijuana, volatile agents, and cocaine. Evidenced-based behavioral treatments account for the large majority of work with pregnant women at programs such as UNC Horizons.
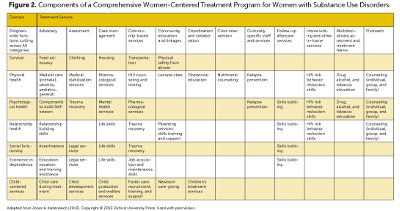
General medication treatments include utilizing the nicotine patch for smoking cessation or naltrexone for alcohol cessation. Disulfiram for alcohol cessation is contraindicated during pregnancy. The aforementioned treatments, although promising, require further research and refinement in use with pregnant women with substance use disorders.
Medications for the treatment of opioid use disorders, however, have demonstrated effectiveness for use with pregnant women with substance use disorders. Opioid agonists (methadone and buprenorphine) and opioid antagonists (naltrexone) are medications that can stabilize pregnant women with opioid use disorders and prevent relapse to opioid use if taken as prescribed and in adequate doses. Such medications are central, but not stand-alone treatments. Opioid agonist pharmacotherapy for pregnant women with opioid use disorders is discussed in detail by Jones and colleagues (2009), as is opioid antagonist pharmacotherapy in pregnancy (Jones, Chisolm, Jansson, & Terplan, 2013).
Treatment Planning for Angela Smith
Angela underwent a comprehensive psychosocial and behavioral screening and assessment at intake, including key elements of the Addiction Severity Index (ASI). Following admission to the outpatient program of Horizons, Angela was required to attend on a daily basis. Because she had no transportation, transportation was provided to and from Horizons on a daily basis on a van whose operation is funded by Horizons. Based on the information Angela provided, she was seen by an obstetric/gynecological staff member for an evaluation of her pregnancy status, and tested for HIV and HCV. She was seen by a psychiatrist for an evaluation not only of her substance use and possible treatment with an opioid agonist for her heroin use, but also of possible co-occurring mental disorders.
Psychological services focused on both psychoeducation and counseling. Angela was enrolled in weekly groups for education regarding drug and alcohol education, relapse prevention for opioid use and cessation of marijuana use, healthy lifestyles where HIV and STI risk reduction skills were covered, parenting skills, and education regarding healthy relationships. She met with a nurse practitioner who addressed smoking cessation program based on the 4As (asking, advising, assisting, arranging) protocol (Glynn, Manley, & Pechacek, 1990), discussed family planning options after she gives birth, and provided basic nutritional counseling. Given that she had a physically abusive boyfriend, she engaged in the trauma-specific group and individual counseling regarding partner violence and unsafe sex practices, with an emphasis on violence prevention and safety planning.
Given Angela’s active attempts to regain custody of her daughter Ellen, she was provided access to legal aid services that had as its goal family reunification. Angela was provided guidance in contacting child protective services, and advice regarding the necessary steps that would be required to regain custody of Ellen.
Child and parenting services focused on both parenting and relationship skills that Angela would find necessary when she regained custody of Ellen, as well as on the parenting skills that Angela would need with the birth of her third child while enrolled at Horizons. A related point of attention was on breastfeeding education, focused both on how best to bond with her child following delivery, as well as on issues and problems with substance use during breastfeeding. In Angela’s case, she was provided information about alcohol use and breastfeeding, the dangers of smoking either cigarettes or marijuana while breastfeeding, and the fact that buprenorphine is compatible with breastfeeding. Additionally, she was told that she should avoid any use of nonprescribed opioids during the time she was breastfeeding.
Finally, given a limited job history, Angela was provided help with developing her resume, applying for jobs, interviewing for jobs, and negotiating employment opportunities.
Angela Postpartum
Twenty-five weeks after admission to Horizons, Angela delivered a healthy, term daughter named Sarah. Angela relapsed to heroin use on two occasions during her prenatal period. She promptly reported her lapses to her counselor, and more intensive counseling was provided to her. She was able to cut down both her cigarette smoking and marijuana use, but not able to sustain abstinence from either substance for more than a month at a time. She reported that she abstained from the use of alcohol during her time at Horizons, which was confirmed with regular observed urine drug screens. She has not yet been able to regain custody of Ellen, but continues to work towards that goal. With the help of social services and her case manager, she has been able to move out of her boyfriend’s apartment, and now lives in low-income housing. Her job prospects remain limited, but she is hoping to enroll in a four-week course at the local community college to help her become an entry-level office manager.
References
Bada, H. S., Bann, C. M., Whitaker, T. M., Bauer, C. R., Shankaran, S., Lagasse, L., . . . Higgins, R. (2012). Protective factors can mitigate behavior problems after prenatal cocaine and other drug exposures. Pediatrics, 130(6), 1479–88.
Chasnoff, I. J., Burns, W. J., Schnoll, S. H., & Burns, K. A. (1985). Cocaine use in pregnancy. New England Journal of Medicine, 313(11), 666–9.
Falgreen Eriksen, H. L., Mortensen, E. L., Kilburn, T., Underbjerg, M., Bertrand, J., Støvring, H., . . . Kesmodel, U. S. (2012). The effects of low to moderate prenatal alcohol exposure in early pregnancy on IQ in five-year-old children. BJOG, 119(10), 1191–1200.
Fallone, M. D., LaGasse, L. L., Lester, B. M., Shankaran, S., Bada, H. S., & Bauer, C. R. (2014). Reactivity and regulation of motor responses in cocaine-exposed infants. Neurotoxicology & Teratology, 43, 25–32.
Fried, P. A. (1995). Prenatal exposure to marihuana and tobacco during infancy, early, and middle childhood: Effects and an attempt at synthesis. Archives of Toxicology Supplement, 17, 233–60.
Glynn, T. J., Manley, M. W., & Pechacek, T. F. (1990). Physician-initiated smoking cessation program: The National Cancer Institute trials. Progress in Clinical and Biological Research, 339, 11–25.
Huizink, A. C. (2014). Prenatal cannabis exposure and infant outcomes: Overview of studies. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 52, 45–52.
Hurt, H., Betancourt, L. M., Malmud, E. K., Shera, D. M., Giannetta, J. M., Brodsky, N. L., & Farah, M. J. (2009). Children with and without gestational cocaine exposure: A neurocognitive systems analysis. Neurotoxicology & Teratology, 31(6), 334–41.
Jones, H. E., & Kaltenbach, K. (2013). Treating women with substance use disorders during pregnancy: A comprehensive approach to caring for mother and child. New York, NY: Oxford University Press.
Jones, H. E., Heil, S. H., Baewert, A., Arria, A. M., Kaltenbach, K., Martin, P. R., . . . Fischer, G. (2012). Buprenorphine treatment of opioid-dependent pregnant women: A comprehensive review. Addiction, 107(Suppl.1), 5–27.
Jones, H. E., Kaltenbach, K., Coyle, M. G., Heil, S. H., O’Grady, K. E., Arria, A. M., . . . Martin, P. R. (2009). Rebuilding lives: Helping women recover from opioid addiction during pregnancy. Counselor, 10(5), 10–9.
Jones, H. E., & Balster, R. L. (1998). Inhalant abuse in pregnancy. Obstetrics and Gynecology Clinics of North America, 25(1), 153–67.
Jones, H. E., Chisolm, M. S., Jansson, L. M., & Terplan, M. (2013). Naltrexone in the treatment of opioid-dependent pregnant women: The case for a considered and measured approach to research. Addiction, 108(2), 233–47.
Kesmodel, U. S., Bertrand, J., Støvring, H., Skarpness, B., Denny, C. H., & Mortensen, E. L. (2012a). The effect of different alcohol drinking patterns in early to mid pregnancy on the child’s intelligence, attention, and executive function. BJOG, 119(10), 1180–90.
Kesmodel, U. S., Eriksen, H. L., Underbjerg, M., Kilburn, T. R., Støvring, H., Wimberley, T., & Mortensen, E. L. (2012b). The effect of alcohol binge drinking in early pregnancy on general intelligence in children. BJOG, 119(10), 1222–31.
Mei-Dan, E., Walfisch, A., Weisz, B., Hallak, M., Brown, R., & Shrim, A. (2014). The unborn smoker: Association between smoking during pregnancy and adverse perinatal outcomes. Journal of Perinatal Medicine.
MyAHEC. (2014). North Carolina area health education centers. Retrieved from http://my.ncahec.net/
National Institute on Drug Abuse (NIDA). (1996). National pregnancy and health survey: Drug use among women delivering livebirths: 1992. Washington, DC: US Department of Health and Human Services. Retrieved from http://www.icpsr.umich.edu/icpsrweb/SAMHDA/studies/2835
Patrick, S. W., Schumacher, R. E., Benneyworth, B. D., Krans, E. E., McAllister, J. M., & Davis, M. M. (2012). Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA, 307(18), 1934–40.
Skogerbø, Å., Kesmodel, U. S., Wimberley, T., Støvring, H., Bertrand, J., Landrø, N. I., & Mortensen, E. L. (2012). The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in five-year-old children. BJOG, 119(10), 1201–10.
Substance Abuse and Mental Health Services Administration (SAMHSA). (2013). Results from the 2012 National Survey on Drug Use and Health: Volume I, summary of national findings. Rockville, MD: Author.
Substance Abuse and Mental Health Services Administration (SAMHSA). (2014a). Fetal alcohol spectrum disorders center for excellence. Retrieved from http://www.fasdcenter.samhsa.gov/
Substance Abuse and Mental Health Services Administration (SAMHSA). (2014b). Results from the 2013 National Survey on Drug Use and Health: Volume I, summary of national findings. Rockville, MD: Author.
Tortoriello, G., Morris, C. V., Alpar, A., Fuzik, J., Shirran, S. L., Calvigioni, D., . . . Harkany, T. (2014). Miswiring the brain: Delta9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO Journal, 33(7), 668–85.
Underbjerg, M., Kesmodel, U. S., Landrø, N. I., Bakketeig, L., Grove, J., Wimberley, T., . . . Mortensen, E. L. (2012). The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on selective and sustained attention in five-year-old children. BJOG, 119(10), 1211–21.
US Department of Health and Human Services. (2009). Treatment Episode Data Set — Admissions (TEDS-A), Concatenated 1992 to present. Retrieved from http://dataarchives.ss.ucla.edu/da_catalog/da_catalog_titleRecord.php?studynumber=M643V1
World Health Organization (WHO). (2014). Guidelines for the identification and management of substance use and substance use disorders in pregnancy. Retrieved from http://apps.who.int/iris/bitstream/10665/107130/1/9789241548731_eng.pdf
Previous Article
Field Reports








 Counselor Magazine is the official publication of the California Association of Addiction Programs and Professionals (CCAPP). Counselor offers online continuing education, article archives, subscription deals, and article submission guidelines. It has been serving the addiction field for more than thirty years.
Counselor Magazine is the official publication of the California Association of Addiction Programs and Professionals (CCAPP). Counselor offers online continuing education, article archives, subscription deals, and article submission guidelines. It has been serving the addiction field for more than thirty years.